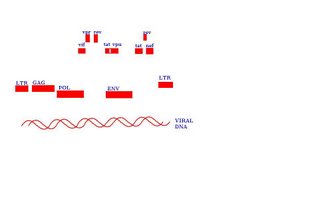
The HIV genome is made up of three genes that code for inner core proteins, envelope proteins and functional proteins (i.e. viral proteins). These are named gag (group antigen-since the antigenicity of this inner core is conserved through out the group, env (envelope) and pol (polymerase) gene which codes for Reverse Transcriptase which has both polymerase and ribonuclease activity. It also codes for other enzymes like integrase whose function is to facilitate the integration of viral DNA into the host cell chromosome and protease which cleaves precursor proteins into smaller functional fragments-the envelope glycoprotein is made as gp160 but is cleaved to form gp120 and gp41.
As with other retroviruses, HIV has at the end of its genome a segment of nucleic acid called LTR (Long Terminal Repeats) which are stretches of DNA that do not by itself code for any protein but functions as a regulator to the expression of the three structural genes, a function that is augmented by other genes called regulatory genes.
Regulatory Genes
These regulate the production of viral proteins: one regulator speeds up protein synthesis, another speeds the production of only some kinds of proteins and a third represses protein synthesis. Each regulatory gene encodes a protein that interacts specifically with a responsive element which is a sequence of nucleotides within the genome. Regulatory genes act in trans because they exert at a distance, the responsive sequence affect adjacent genes hence act in cis. Through their influence regulatory genes can effect an explosive viral replication, moderate growth or quiescence.
The tat Gene
That tat gene (Trans-activator of transcription) is both unusual in both structure and effect; it occurs as two widely separated sequences of nucleotides. The gene starts from nucleotide 5831 to 6045 and then 8379 to 8424.
This gene is translated to a polypeptide called Tat. This protein when bound to the viral RNA boost the expression of viral genes. It interacts with a kinase that is involved in the phosphorylation of the carboxy-terminal which encode a protein which when bound to viral RNA boost the expression of viral gene domain (CTD) of RNA polymerase-II required for initial transcription. To exert its effect the tat protein is dependent upon a short sequence of nucleotides known as TAR (trans-activating responsive sequence). Tat stimulatory effect extend to all viral proteins, it also positively feeds back upon itself resulting in enormous synthesis of viral proteins. It activates the expression of TNF-β, TGF-β but down-regulate other cellular gene expression including bcl-2 and the chemokine MIP-1α.
The rev Gene
A second regulator gene rev (regulator of expression of viral-protein) also exists as two widely separated nucleotide sequence, one from 5 970 to 6 045 then another from 8 379 to 8 653. The gene encodes a 13kD protein Rev which enables the integrated virus to produce selectively either regulatory proteins or virion components. It acts as a genetic switch whose role is in the activation of the virus from latency to active viral replication.
The vpr Gene
The gene is found between the 5 559th and 5 795th nucleotide. It encodes a Vpr protein. The protein is incorporated into viral particle. Approximately 100 copies of vpr are associated with each virion. This is mediated through specific interaction between the carboxyl terminal region of p55 gag which corresponds to p6 in the proteolytically processed protein. It confers the ability of HIV-1 to infect non-dividing cells by facilitating nuclear localisation of the pre-integration complex. This NL is distinct from the prototypic NLS because there is no negative feedback, but the NLS peptide can inhibit the nuclear localisation of other proteins containing conventional NLS eg matrix (p17) protein. It also affect cell division- all cells expressing vpr accumulate in the G2 phase of the cell cycle; vpr expression prevents activity of p34cdc2/cyclin B complex an activator of the cell cycle important for entry into mitosis.
The vpu gene (6062-6310)
Vpu codes for a 16kD polypeptide- an integral membrane phosphoprotein localised in the internal membrane of the cell. It is expressed from mRNa that also encodes for envelope proteins but translated at levels ten times lower than that of env. A complex gp120/CD4 occurs in the endoplasmic reticulum but vpu degrades the CD4 molecule because this complex interferes with virion assembly.
The vif Gene (5 041- 5 619)
Codes for a 23kD protein which is responsible for the replication of HIV in the peripheral blood lymphocytes & macrophages. Other cells have complimentary proteins to vif hence not needed.
The nef Gene (8 797-9168)
The Nef gene (negative –regulator factor) encodes a 27kD myristylated protein which is the first viral protein to accumulate to detectable levels in cells following HIV-1 infection. The protein down-regulates the expression of CD4 by infected cells by stimulating CD4 endocytosis and lysosomal degradation by interaction with the dileucine repeat sequence contained in its membrane proximal region. Also down-regulates cell-surface expression of MHC-1, this is the protein that is responsible for the perturbation of T-cell activation and stimulation of infectivity.
© freeman Chari 2005